Master thesis: Investigation of Strategies for Implementing Automation in the Quality Management System of a Medical Device Company
Master thesis by Clara Piris, Quality and Regulatory Affairs Specialist at cosinuss°
In today’s fast-evolving regulatory landscape, medical device companies, like cosinuss°, face growing demands for compliance, data accuracy, and process efficiency. Post-Market Surveillance (PMS) – the ongoing monitoring of product safety and performance after market launch – is one of the most complex and resource-intensive components of a Quality Management System (QMS). This challenge is particularly pressing for smaller companies, where resources are often limited, yet regulatory expectations remain high.
Focus: Enhancing QMS processes by automation
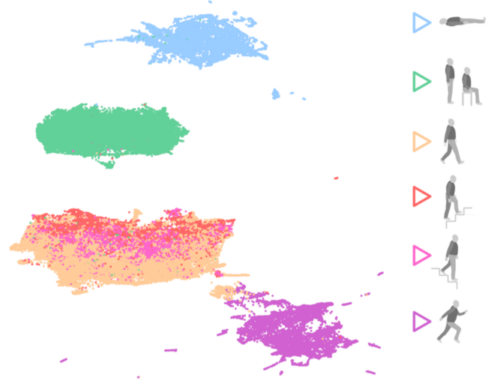
This study investigated how automation can enhance QMS processes, with a special focus on PMS. Through hands-on implementation within the company’s quality framework, the research examined how digital tools, structured databases, and automated workflows can reduce manual workload, improve data quality, and strengthen compliance with international standards such as the MDR, ISO 13485, and FDA guidelines.
Method: What Was Done
The master thesis focused on introducing automation across key QMS functions, including:
- Risk management and design traceability
- Complaint and non-conformance tracking
- KPI monitoring and reporting
- Centralized data management
By replacing spreadsheets and manual documentation with integrated, automated systems, the thesis demonstrated how quality processes can become more reliable, transparent, and scalable.
Key Outcomes: Clear benefits through automation
The implementation of these strategies delivered clear benefits:
- Stronger regulatory alignment through traceable, audit-ready systems
- More efficient workflows, saving time and reducing human error
- Improved decision-making, thanks to real-time data and automated reporting
- A scalable framework that supports sustainable growth and compliance
- Reduced manual workload for the team
Conclusion: Strategic tool for medical device companies
The master thesis shows how automation is not just a technological upgrade – it’s a strategic tool for building resilient, future-ready quality systems. The insights gained offer a roadmap for cosinuss° – and other medical device companies – looking to modernize QMS while staying agile and compliant in an increasingly regulated world.